 Introduction
Introduction
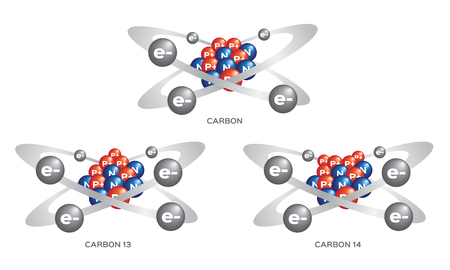
Studies of radioactivity at the beginning of the 20th century made it possible to investigate the actual structure and mass of atoms. Gradually, evidence began to build that atoms of the same element could have different masses. These atoms were called isotopes. How are isotopes distinguished from one another? What is the average atomic mass of an element that has different isotopes?
The purpose of this activity is to investigate the mass properties and relative abundance of isotopes for the “bean bag” element (symbol, Bg) and to calculate the atomic mass of this element.
Click here to download the complete activity


 Introduction
Introduction